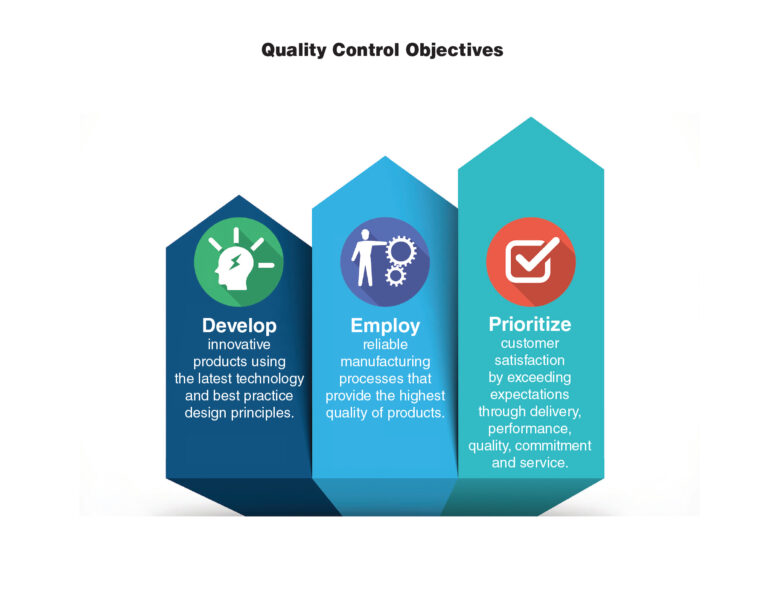
Quality assurance at The Lee Company is a management-driven system for excellence in product quality and service. The objective of the quality management system is to ensure that our products and services meet or exceed the customer’s quality expectations.
We strive to continually improve the effectiveness of the quality management system through our quality policy, quality objectives, audit results, analysis of data, corrective and preventative action plans, management reviews, and other continuous improvement initiatives. Through our management system, we are committed to upholding the high standards of quality as well as the safety, education, and well-being of our employees and our environment.
The Precision Microhydraulics Quality Management System is based on AS9100, and the Electro-Fluidic Systems Quality Management System is based on ISO 9001, and the Industrial Microhydraulics Quality Management System complies with the requirements of IATF 16949. Our quality systems are supported by a documented lot control and traceability system which creates a framework for clearly defining the control of materials, processes, and verification activities, thus providing our customers with the confidence that the design and manufacture of our products are performed in a well-defined and controlled environment.
These quality systems, which include the extensive use of Statistical Process Control and risk mitigation methods have enabled The Lee Company to be awarded Certified Supplier status by many of our customers. This allows those companies to receive Lee products directly into stock, saving the costs and delays associated with incoming inspection. Quality Assurance surveys, audits, and source inspections are always welcomed by Lee.
Always verify flow calculations by experiment.
*There are many parameters to consider when determining V-Factor. Click here for more information.